About the Costerton Biofilm Center
The Costerton Biofilm Center is a unique interdisciplinary research center established to explore the field of chronic infections caused by bacteria.

The Costerton Biofilm Center is named in the honour of Dr John William Costerton.
Dr John William (Bill) Costerton studied biofilms for more than 40 years. As a Canadian, he was a man of the mountains and spent hours climbing in his beloved Canadian Rockies. His PhD was in microbiology and he wanted to combine his passion for his two hobbies - climbing and science.
 His first observation was when he tackled an alpine stream in the Bugaboos in eastern British Columbia, Canada. While climbing he noticed the rocks were slippery and he observed that whereas the free floating water contained only few bacteria per milliliter the slippery rocks were covered with bacteria stuck in “slime”.
His first observation was when he tackled an alpine stream in the Bugaboos in eastern British Columbia, Canada. While climbing he noticed the rocks were slippery and he observed that whereas the free floating water contained only few bacteria per milliliter the slippery rocks were covered with bacteria stuck in “slime”.
Biofilm paradigm
Dr. Costerton pioneered the development of the biofilm theory of which bacteria grow enclosed in a protective, biopolymeric matrix forming a film that is adherent to solid surfaces (a sessile organism). Together with University of Copenhagen's Dr. Niels Høiby, he strongly promoted the view that bacteria in biofilms (environmental as well as medical relevant) differ significantly from their planktonic (free-floating) counterparts (Costerton et al., Sci Am 1978).
Dr. Costerton published over 700 peer-reviewed papers that provide a solid basis for the understanding of bacterial processes in environmental, dental and medical microbiology. The research led to many industrial and medical relevant breakthroughs, confirming that biofilms cause chronic infections and biofilm implant-related infections represent one of the most difficult-to-treat infections in humans.
Dr. John William Costerton passed away on 12 May 2012.
The Costerton Biofilm Center is a unique interdisciplinary research center established to explore the field of chronic infections caused by bacteria. In recent years it has been realized that the ability of bacteria to form biofilm contributes significantly to such infections which are becoming a major health challenge with large global economic and societal implications. In biofilm bacteria are located in densely packed, slow growing microcolonies concealed in a self-produced protective matrix of biopolymers. In this life-mode, the bacteria attain the highest levels of resistance to our present assortment of antibiotics and our immune system.
The center aims at the delivery of new approaches to address key challenges within medical biofilm research in order to generate fundamental insights into the biology of chronic infections. This ambitious goal requires novel integrative approaches that involve the joint effort of leading researchers from complementary research fields.
The activities include basic research in order to generate cutting edge and profound knowledge about medical, relevant biofilms. The knowledge gained forms the basis for the development of new means of diagnosis, prevention and treatment of bacterial infections with the intent of improved public health and economic gains.
Objective
The overall objective is to elucidate molecular mechanisms of biofilm formation, and identify novel diagnostic methods and bioactive chemicals to guide the development of biofilm controlling drugs that can help reinstate proper action of antibiotics and the immune system, and device efficient treatment scenarios of chronic infections.
In nature bacteria often appear as sessile, matrix embedded aggregates commonly referred to as biofilms. There is accumulating evidence that in case bacteria succeed in forming biofilms within the host, the infection develops into a chronic state. The important hallmarks are development of inflammations, host tissue degradation, extreme tolerance to the action of conventional antimicrobial agents and an almost infinite capacity to evade the host defense systems. With an apparent similarity to cancers, biofilms can proliferate into a dispersal mode, spread cells and spark new areas of biofilm infections.
Biofilms and procedures of modern medicine
Due to advances in medical technology and an ageing population, there is an increasing problem with medical device-associated infections. In recent years it has been realized that the ability of bacteria to form biofilm contributes significantly to antibiotic resistance. In biofilms bacteria are located in densely packed, slow growing microcolonies concealed in a self-produced protective matrix of biopolymers. In this life-mode, the bacteria attain the highest levels of resistance to our present assortment of antibiotics and our immune system. Accordingly, biofilms are a common cause of persistent infections. Biofilm-based infections are a major socio-economic burden implicating hospitalization, patient suffering, reduced life quality and lost employment. It is estimated that about 60–70% of hospital acquired infections are caused by bacterial biofilms.
The bacteria that are involved in hospital acquired infections are primarily Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp., which are denoted by their acronym ESKAPEE, and declared by WHO and other agencies to be a top global threat.
The World Health Organization (WHO), United Nations (UN) and Center for Disease Control and Prevention (CDC) predict that the number of antibiotic resistant (referred to as AMR) infections will rise to unmanageable levels in all parts of the world if adequate and appropriate countermeasures are not taken. It is projected that deaths attributable to resistant infections will reach 10 million per year by 2050, if new antibiotics are not developed. Poor antibiotic efficacy against AMR infections has broad and deep health impacts, as well as severe social and economic implications. This includes severely constrained therapy options with the loss of the safety-net that antibiotics provide for people undergoing a wide range of modern medical procedures such as surgery, insertion of medical devices, and cancer therapy.
Integrating medical-, oral-, and environmental- microbiology with development of biofilm controlling chemical agents will pave the way for a solution to combat the problem of biofilm induced infections.
Potential biofilm locations
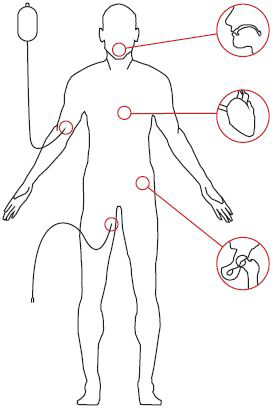
- Dental plaque
- Coronary implants
- Venous catheter
- Hip implant
- Urinary catheter
A tribute in memory of our colleague and friend Professor Paul Stoodley
Paul Stoodley held the positions of Professor of Microbial Infection and Immunity and Orthopedics at the Ohio State University, and Professor in Microbial Tribology at University of Southampton, among several other academic and research appointments and responsibilities, in the US, UK and internationally.

Paul was a key international research leader in the field of microbial biofilms, across fundamental science and the numerous profound applications of this scientific field. On the basis of introducing new multidisciplinary research programs, Paul led the way and expanded the scope, capacities, and excellence of research at several microbial biofilm research centres across the globe. Indeed, Paul was an exemplar leader, inventor, mentor and scientific ambassador.
Microbes are everywhere and have the unique capacity to form complex assemblages, which we recognize as microbial biofilms, on all surfaces in all natural as well as engineered systems. Their presence and actions have profound outcomes, and studying, understanding and addressing such systems are complex and require cross disciplinary skills. This is reflected by the establishment of national and international leading research centres with unique research expertise as based on the embedment of emerging disciplines and fostering novel cross-disciplinary research efforts.
Paul had unique multidisciplinary skills across engineering, biophysics, microbiology and numerous other sciences, combined with the capacity to engage and communicate science to any audience. These qualities made Paul one of the most influential thought leaders and drivers of the uptake of biofilm sciences, both nationally and internationally. This development included numerous fields, encouraged excellence in both basic and applied research, and significantly aided the development of biofilm research centers and entities of thematic scientific and educational programs now operational in many countries and organizations, both within university settings and beyond.
Paul not only shaped the biofilm field but was a wonderful and supportive colleague and friend to many. He was a thinker and a leader, and one of strongest pillars, socially and scientifically, of all biofilm conferences organized across the world. Our thoughts also go to his wife Luanne and his family, friends and lab members. Paul was a generous man and will be greatly missed.
On behalf of Paul’s colleagues and friends at the Singapore Centre for Environmental Life Sciences Engineering (SCELSE), Singapore; the National Biofilms Innovation Centre (NBIC), UK; Aarhus University Centre for Water Technology (WATEC), Denmark; and the Costerton Biofilm Center (CBC), Denmark.
Alain Filloux & Staffan Kjelleberg (SCELSE); Jeremy Webb & Jo Slater-Jefferies (NBIC); Rikke Meyer &
Tom Seviour (WATEC); Michael Givskov & Thomas Bjarnsholt (CBC).
Directors address

The Costerton Biofilm Center has become a unique, internationally recognized, interdisciplinary research hub dedicated to advancing knowledge in chronic bacterial infections under the guidance of Michael Givskov. I am honored to carry the CBC torch forward now.
Effective 1 October 2025, Professor Michael Givskov has stepped down as Director of the Costerton Biofilm Center (CBC). Professor Thomas Bjarnsholt now assumes the role.

Michael Givskov has played a pivotal role in establishing CBC in 2013. Under his guidance, the Costerton Biofilm Center has become a unique, internationally recognized, interdisciplinary research hub dedicated to advancing knowledge in chronic bacterial infections.
Thomas Bjarnsholt completed his PhD and postdoctoral studies under Michael’s supervision, Thomas went on to build an independent career, becoming Professor at the Department of Immunology and Microbiology and has been an integral member of CBC for many years.
The Costerton Biofilm Center provides a forum for scientists and clinicians and encourages research into the microbial aetiology of biofilms. By integrating translational and clinically relevant research, the Center takes lead in improved prevention and development of new treatments of diseases caused by biofilms. The research aims at explaining the riddle as to why biofilm-bacteria gain the upper hand in the fight against our immune system, and hopefully lead to new and innovative strategies for early diagnosis, treatment and prevention of chronic diseases for the benefit of public health.
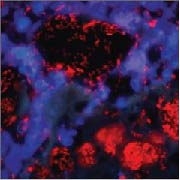
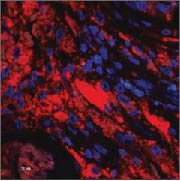
Bacteria are visualized by means of FISH hybridization (red) and immune cells by DAPI stain (blue). The images illustrate the common pattern in two different chronic diseases: aggregates of biofilm bacteria surrounded by numerous immune, inflammatory cells that fail to eliminate the biofilm.
