Molecular Biofilm Biology
The overarching purpose of our research is to understand the mechanistic basis of bacterial biofilm formation and biofilm-associated antimicrobial tolerance, and to use this knowledge to develop novel strategies and drug candidates for prevention and treatment of biofilm-based infections.
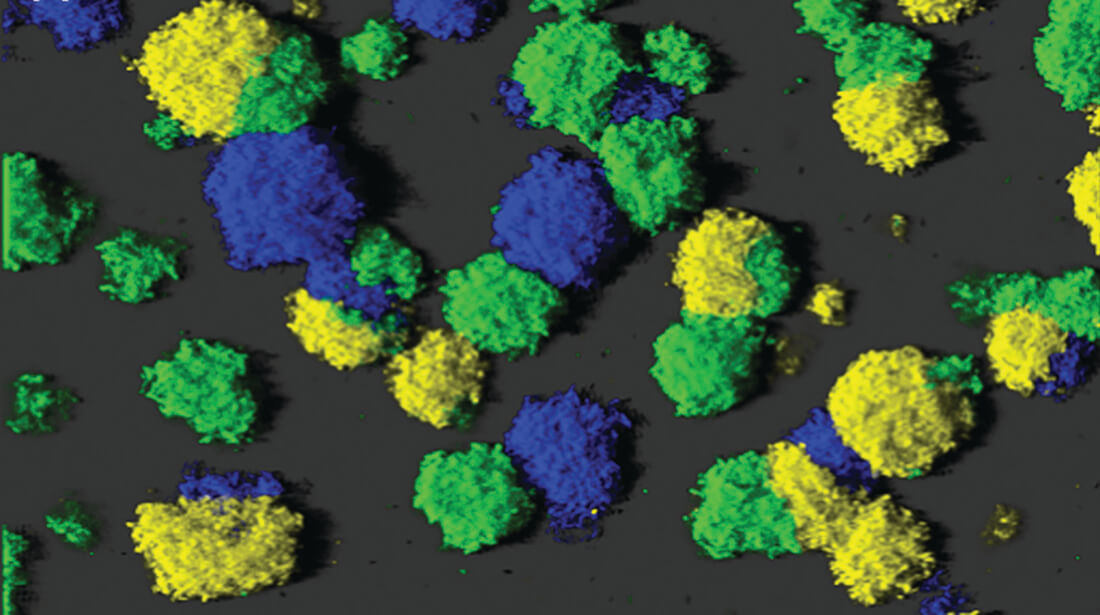
Biofilm formation mechanisms
Biofilms are communities of aggregated bacterial cells embedded in an extracellular polymeric matrix, and they are associated with numerous chronic infections since bacteria in biofilms are tolerant to antimicrobial treatments and host immune defenses. Recent research suggests that the molecule cyclic diguanosine monophosphate (c-di-GMP) constitutes a ubiquitous intracellular regulator of bacterial biofilm formation. High levels of c-di-GMP induce formation of biofilms, whereas low levels of c-di-GMP induce a planktonic bacterial lifestyle. The level of c-di-GMP in bacteria is determined by diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) which contain catalytic GGDEF and EAL/HD-GYP domains as well as regulatory domains. The research project is aimed at obtaining knowledge of the regulation of the activity of DGCs and PDEs, as well as on identification of down-stream c-di-GMP affecter proteins. This knowledge may reveal novel molecular targets for new drugs that can prevent or cure biofilm infections.
Funding: The European Research Council
Mechanisms of biofilm-associated antimicrobial tolerance
It is well established that bacterial biofilms are more tolerant to antibiotics than planktonic bacteria. Several factors are known to play a role in the high antimicrobial tolerance displayed by biofilms, including restricted antibiotic penetration, physiological heterogeneity, and induction of specific antibiotic resistance genes. However, our current knowledge is fragmented and the relative importance of the different factors is not known. Accordingly, more knowledge is warranted about antibiotic tolerance mechanisms in order for us to develop effective treatments against biofilm infections. In a collaboration with Professor Leo Eberl at the University of Zürich, we use the next-generation DNA sequencing technique Tn-Seq to identify the complete set of genes that play a role in antibiotic tolerance of Pseudomonas aeruginosa and Burkholderia cenecepacia biofilms. Thereby, the proteins and molecular mechanisms underlying antibiotic tolerance can be identified, and new drug targets can be suggested.
Development of anti-biofilm compounds
Biofilms formed by opportunistic pathogenic bacteria can resist immune defenses and antimicrobial therapy, and therefore cause considerable problems in medical settings. However, if forced away from the protective biofilm-state to a free-living planktonic mode, the bacteria become susceptible to the action of the immune system and antimicrobials. Current research indicates that the secondary messenger c-di-GMP is a general controller of the biofilm lifestyle in bacteria. High internal levels of c-di-GMP induce production of extracellular matrix components which promote biofilm formation, whereas low c-di-GMP levels down-regulate the production of extracellular matrix components and lead bacteria to the planktonic mode of life. We use a high-throughput screening approach for identification of compounds that can reduce the c-di-GMP content in bacteria, and prevent biofilm formation/disperse biofilms. Such compounds are expected to serve as lead molecules for the development of drugs that can cure problematic bacterial infections.
Chemical compounds that impede the pathogenic effects of Staphylococcus aureus in atopic dermatitis
Atopic dermatitis (AD) is an inflammatory skin disease that affects up to 5% of adults and 20% of children worldwide. The skin of individuals with AD is often colonized with the bacterium Staphylococcus aureus, and this is associated with exacerbation of the disease. On the other hand, the presence of commensal skin bacteria is known to alleviate the symptoms of AD. Recently, compelling evidence was presented that specifically the Sbi protein produced by S. aureus is responsible for eliciting an immune response that exacerbates the AD disease. This finding suggests the possibility of targeted treatment of AD with minimal disturbance of the commensal skin microbiome. The aim of the research project is to develop chemical compounds that specifically inhibit production of the Sbi protein in S. aureus, and impede the pathogenic effects of S. aureus in atopic dermatitis, without disturbing the beneficial commensal skin bacteria. The project is expected to improve our understanding of the underlying biological, medicinal and pharmacological mechanisms of dermatitis and its symptoms, and to provide lead chemical compounds that potentially can be developed into medicine against AD flares.
Funding: The LEO Foundation
The research projects are currently funded by grants from “The European Research Council, the Novo Nordisk Foundation and the Leo Foundation."
The Tolker-Nielsen research group collaborates with numerous scientists around the world. About half of the scientific publications involve international collaboration.
Staff list
| Name | Title | Phone | |
|---|---|---|---|
| Goltermann, Lise | Assistant Professor | +4535335550 | |
| Lin, Shiyu | Research Fellow | ||
| Mørk, Louise | Laboratory Technician | +4535336603 | |
| Rybtke, Morten Levin | Assistant Professor | +4535329573 | |
| Shahryari, Shahab | Postdoc | +4535331045 | |
| Tolker-Nielsen, Tim | Professor | +4535337373 |


